Preparation of barium
Industrial preparation of metallic barium includes two steps: preparation of barium oxide and preparation of metallic barium by metal thermal reduction (aluminothermic reduction).
| Product | Barium | ||
| CAS No | 7647-17-8 | ||
| Batch No. | 16121606 | Quantity: | 100.00kg |
| Date of manufacturing: | Dec,16,2016 | Date of test: | Dec,16,2016 |
| Test Item w/% | Results | Test Item w/% | Results |
| Ba | >99.92% | Sb | <0.0005 |
| Be | <0.0005 | Ca | 0.015 |
| Na | <0.001 | Sr | 0.045 |
| Mg | 0.0013 | Ti | <0.0005 |
| Al | 0.017 | Cr | <0.0005 |
| Si | 0.0015 | Mn | 0.0015 |
| K | <0.001 | Fe | <0.001 |
| As | <0.001 | Ni | <0.0005 |
| Sn | <0.0005 | Cu | <0.0005 |
| Test Standard | Be, Na and other 16 elements: ICP-MS
Ca, Sr: ICP-AES Ba: TC-TIC |
||
| Conclusion: |
Comply to the enterprise standard |
||

(1) Preparation of barium oxide
High-quality barite ore must first be hand-selected and floated, and then iron and silicon are removed to obtain a concentrate containing more than 96% barium sulfate. The ore powder with a particle size of less than 20 mesh is mixed with coal or petroleum coke powder in a weight ratio of 4:1, and roasted at 1100℃ in a reverberatory furnace. The barium sulfate is reduced to barium sulfide (commonly known as "black ash"), and the obtained barium sulfide solution is leached with hot water. In order to convert barium sulfide into barium carbonate precipitation, sodium carbonate or carbon dioxide needs to be added to the barium sulfide aqueous solution. Barium oxide can be obtained by mixing barium carbonate with carbon powder and calcining it at above 800℃. It should be noted that barium oxide is oxidized to form barium peroxide at 500-700℃, and barium peroxide can be decomposed to form barium oxide at 700-800℃. Therefore, in order to avoid the production of barium peroxide, the calcined product needs to be cooled or quenched under the protection of inert gas.
(2) Aluminothermic reduction method to produce metallic barium
Due to different ingredients, there are two reactions of aluminum reducing barium oxide:
6BaO+2Al→3BaO•Al2O3+3Ba↑
Or: 4BaO+2Al→BaO•Al2O3+3Ba↑
At 1000-1200℃, these two reactions produce very little barium, so a vacuum pump is needed to continuously transfer the barium vapor from the reaction zone to the condensation zone so that the reaction can continue to proceed to the right. The residue after the reaction is toxic and needs to be treated before it can be discarded.
Preparation of common barium compounds
(1) Preparation method of barium carbonate
① Carbonization method
The carbonization method mainly involves mixing barite and coal in a certain proportion, crushing them into a rotary kiln and calcining and reducing them at 1100-1200℃ to obtain a barium sulfide melt. Carbon dioxide is introduced into the barium sulfide solution for carbonization, and the reaction is as follows:
BaS+CO2+H2O=BaCO3+H2S
The obtained barium carbonate slurry is desulfurized, washed and vacuum filtered, and then dried and crushed at 300℃ to obtain a finished barium carbonate product. This method is simple in process and low in cost, so it is adopted by most manufacturers.
② Double decomposition method
Barium sulfide and ammonium carbonate undergo double decomposition reaction, and the reaction is as follows:
BaS+(NH4)2CO3=BaCO3+(NH4)2S
Or barium chloride reacts with potassium carbonate, and the reaction is as follows:
BaCl2+K2CO3=BaCO3+2KCl
The product obtained from the reaction is then washed, filtered, dried, etc. to obtain a finished barium carbonate product.
③ Barium carbonate method
Barium carbonate powder is reacted with ammonium salt to generate soluble barium salt, and ammonium carbonate is recycled. Soluble barium salt is added to ammonium carbonate to precipitate refined barium carbonate, which is filtered and dried to make the finished product. In addition, the mother liquor obtained can be recycled. The reaction is as follows:
BaCO3+2HCl=BaCl2+H2O+CO2
BaCl2+2NH4OH=Ba(OH)2+2NH4Cl
Ba(OH)2+CO2=BaCO3+H2O
(2) Preparation method of barium titanate
① Solid phase method
Barium titanate can be obtained by calcining barium carbonate and titanium dioxide, and any other materials can be doped into it. The reaction is as follows:
TiO2 + BaCO3 = BaTiO3 + CO2↑
② Coprecipitation method
Barium chloride and titanium tetrachloride are mixed and dissolved in equal amounts, heated to 70°C, and then oxalic acid is added dropwise to obtain hydrated barium titanyl oxalate [BaTiO(C2O4)2•4H2O] precipitate, which is washed, dried, and then pyrolyzed to obtain barium titanate. The reaction is as follows:
BaCl2 + TiCl4 + 2H2C2O4 + 5H2O = BaTiO(C2O4)2•4H2O↓ + 6HCl
BaTiO(C2O4)2•4H2O = BaTiO3 + 2CO2↑ + 2CO↑ + 4H2O
After beating the metatitanic acid, a barium chloride solution is added, and then ammonium carbonate is added under stirring to generate a coprecipitate of barium carbonate and metatitanic acid, which is calcined to obtain the product. The reaction is as follows:
BaCl2 + (NH4)2CO3 = BaCO3 + 2NH4Cl
H2TiO3 + BaCO3 = BaTiO3 + CO2↑ + H2O
(3) Preparation of barium chloride
The production process of barium chloride mainly includes hydrochloric acid method, barium carbonate method, calcium chloride method and magnesium chloride method according to the different methods or raw materials.
① Hydrochloric acid method. When barium sulfide is treated with hydrochloric acid, the main reaction is:
BaS+2HCI=BaCl2+H2S↑+Q
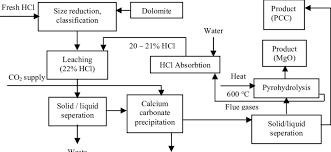
②Barium carbonate method. Made with barium carbonate (barium carbonate) as raw material, the main reactions are:
BaCO3+2HCI=BaCl2+CO2↑+H2O
③Carbonization method

Effects of barium on human health
How does barium affect health?
Barium is not an essential element for the human body, but it has a great impact on human health. Barium may be exposed to barium during barium mining, smelting, manufacturing, and use of barium compounds. Barium and its compounds can enter the body through the respiratory tract, digestive tract, and damaged skin. Occupational barium poisoning is mainly caused by respiratory inhalation, which occurs in accidents during production and use; non-occupational barium poisoning is mainly caused by digestive tract ingestion, mostly caused by accidental ingestion; liquid soluble barium compounds can be absorbed through wounded skin. Acute barium poisoning is mostly caused by accidental ingestion.
Medical use
(1) Barium meal radiography
Barium meal radiography, also known as digestive tract barium radiography, is an examination method that uses barium sulfate as a contrast agent to show whether there are lesions in the digestive tract under X-ray irradiation. Barium meal radiography is an oral ingestion of contrast agents, and the medicinal barium sulfate used as a contrast agent is insoluble in water and lipids and will not be absorbed by the gastrointestinal mucosa, so it is basically non-toxic to humans.

According to the needs of clinical diagnosis and treatment, gastrointestinal barium meal radiography can be divided into upper gastrointestinal barium meal, whole gastrointestinal barium meal, colon barium enema and small intestinal barium enema examination.
Barium poisoning
Routes of exposure
Barium can be exposed to barium during barium mining, smelting, and manufacturing. In addition, barium and its compounds are widely used. Common toxic barium salts include barium carbonate, barium chloride, barium sulfide, barium nitrate, and barium oxide. Some daily necessities also contain barium, such as barium sulfide in hair removal drugs. Some agricultural pest control agents or rodenticides also contain soluble barium salts such as barium chloride and barium carbonate.
Post time: Jan-15-2025